|
- Current Therapeutic Research Vol.58,No.12,December 1997 - Multicenter
Clinical Trial Of The Serum Lipid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Junxian
Wang, Zongliang Lu, Jiamin Chi,
Wenhua Wang, Meishe,
Wenrong Kou, Pulin
Yu, Lijiang Yu, Li Chen,
Jia-Shi Zhu, And Joseph
Chang
Dongzhimen Hospital, Beijing University for Traditional Chinese Medicine, Beijing, Cardiovascular Institute and Fuwai Hospital, Chinese Academy of Medical Sciences, Beijing, Beijing Hospital, Ministry of Public Health of China, Beijing, Clinical Pharmacology Research Center of the Ministry of Public Health at Haerbin Medical University, Haerbin, Heilongjiang, WBL Peking University Biotech. Co. Ltd., Beijing, People's Republic of China, and Pharmanex, Inc., Simi Valley, California |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
INTRODUCTION Hyperlipidemia is a highly predictive risk factor for atherosclerosis, coronary artery disease, and cerebral vascular diseases. 1-3 Regulating hyperlipidemia has proven to be effective in lowering the morbidity and mortality associated with coronary artery disease. 3-5 Among the current lipid-lowering agents, hepatic hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors are effective in reducing total cholesterol (TC) and low-density lipoprotein (LDL) cholesterol. Nonetheless, the high cost of these drugs and their limited availability to the majority of the Chinese popultion have prompted Chinese medical investigators to search for alternative agents to lower serum lipids. Because traditional Chinese medicine, through centuries of empirical use, has produced an extensive library of active herbs, it is reasonable to investigate whether a lipid-lowering dietary supplement could be identified from this library. After an intensive investigation, Monascus purpureus (red yeast)rice was identified as an effective herb to lower serum cholesterol and triglycerides (TG) in animals, and its hypolipidemic effects were confirmed in pilot clinical studies. 6-10 The M purpureus preparation is derived from a strain of M purpureus Went yeast (Xuezhingkang in Chinese ) that is prepared by a traditional rice fermentation method. Red Yeast rice has been used for centuries in China to make rice wine and to flavor foods. It was reported in the ancient Chinese pharmacopoeia, BenCaoGangMu-DanShiBuYi, which was published during the Ming Dynasty (1368-1644), 11to promote blood circulation. Recent studies showed that M purpureus rice contains HMG-CoA reductase inhibitors that reduce the synthesis of cholesterol in the liver. 6,12This herb also contains large quantities of unsaturated fatty acids (>125 mg/g M purpureus rice preparation), including monounsaturated fatty acids, diene-, triene-, tetraene-, and pentaene-fatty acids. Although the function of these unsaturated fatty acids is not known, they may heop reduce serum lipids. 13Other components include proteins, amino acids, saccharides, beta-sitosterol, campesterol, stigmasterol, isoflavone and its glycosides, saponin and sapogenin, and many trace elements.12 The lipid-lowering effects of M purpureus rice have been shown in several animal models of hyperlipidemia to inhibit and prevent increases in TC, LDL-cholesterol, and TG. 6For example, in rabbits fed a diet of 25% casein to induce endogenous hypercholesterolemia, treatment with M purpureus rice for 30 days at daily dosages of 0.4 and 0.8 g/kg significantly lowered serum TC concentration and the TC:high-density lipoprotein (HDL)-cholesterol ratio. 6In a second rabbit model in which hyperlipidemia was induced exogenously by an atherogenic diet, 6oral M purpureus rice preparation prevented increases in serum TC and TG concentrations and the TC: HDL-cholesterol ratio (P<0.05, compared with untreated hyperlipidemic controls). When hyperlipidemia was induced exogenously in quail by feeding an atherogenic diet that included 1% cholesterol, 14% lard, and 6% soybean oil, M purpureus rice preparation administered orally significantly reduced serum TC and TG concentrations. Acute and chronic animal toxicity studies further showed that M purpureus rice is well tolerated, even at doses several times higher than the effective therapeutic dose (manuscript in preparation). In
the present study, we demonstrated that a standardized M purpureus
rice preparation reduced elevated serum cholesterol and TG, as compared
with another herb, Jiaogulan (Gynostemma pentaphylla), which is
believed to be an antihyperlipidemic agent in China.14 This randomized, single-masked trial included a total of 502 patients with a clinical diagnosis of primary hyperlipidemia who were enrolled at four clinical sites in China. Before their blood tests, patients abstained from alcoholic beverages and meals high in fats for 1 day. Patients also discontinued any medication for hyperlipidemia for more than 4 weeks and received dietary advice for 2 to 4 weeks. Eligible patients were recruited if their serum TC was =230 mg/dl (5.95 mmol/L) or higher, LDL-cholesterol was =130 mg/dl (3.41mmol/L), or TG was 200 to 400 mg/dl (2.26 to 4.52 mmol/L). In addition, HDL-cholesterol was =40 mg/dl (1.04 mmol/L) for men or =45 mg/dl (1.16 mmol/L) for women. Patients were excluded from the trial if they had any of the following during the last 6 months: myocardial infarction, stroke, severe trauma or major surgery, nephrotic syndrome, hypothyroidism, acute or chronic hepatobiliary disorders, diabetes mellitus, gout, allergies, or psychosis. Patients who agreed to participate in this clinical trial were informed of the possible benefits and risks. Based on pretreatment TC and TG values, patients were prospectively randomized by a statistician into two unequal arms using a strategy of four-group randomization required by the Chinese Ministry of Public Health for clinical studies of traditional Chinese medicine. 15Groups A, C, and D were the treatment groups, and group B was the positive control group. The equality of the randomization distributions was verified by computer analysis. Study DesignPatients were treated in a single-masked fashion. The treatment group was given a standardized preparation (alcoholic extraction) for M purpureus rice 0.6 g twice a day (1.2 g/d) for 8 weeks. Patients in the positive control group were treated with a traditional Chinese medicine with putative hypolipidemic properties, Jiaogulan, three tablets twice a day (1.2 g/d). Throughout the study, all patients were asked to maintain their normal lifestyles, including smoking, exercise, and dietary habits as advised by physicians 2 to 4 weeks before the trial treatment. All medications were allowed during the trial, except those that could affect serum lipids. Hypolipidemic
Efficacy The overall efficacy of M purpureus rice preparation was also assessed by an integrated analysis of the improvement in serum lipids. The criteria for the assessment of the overall clinical efficacy of M purpureus rice preparation are as follows16:
Patients visited the clinic before the trial and after 4 and 8 weeks of treatment. Patient compliance (determined by counting the remaining pills during clinic visits), general physical health, and symptoms were assessed at each visit. Physical examination (body weight, heart rate and rhythm, liver and spleen palpation), electrocardiogram, complete blood counts, serum alanine aminotransferase, blood urea nitrogen, creatinine, glucose, and creatine kinase, and urinalysis were performed on all patients before and after 8 weeks of therapy. Statistical Analysis Data are analyzed using the SAS statistical software package (SAS Institute, Inc., Cary, North Carolina) expressed as mean±SE. Student's t test was used to analyze the quantitative data, with the group t test for comparisons of data from the two groups and the paired t test for comparisons of continuous variables before and after treatment. A chi-squared test (odds ratio) was used for analyzing the qualitative data and the Breslow-Day test for analyzing homogeneity of odds ratios for the two groups. Regression analysis was used to analyze the relationship between changes in body weight and individual serum lipids. RESULTS A total of 446 (89%) of the 502 enrolled patients completed the study; data from 56 could not be used for analysis for reasons of noncompliance and incomplete data collection. Two patients discontinued treatment because of exacerbation of their preexisting stomachache, and seven patients were determined to be noncompliant because of their inconsistent use of medication. The data for these nine patients were not used for final calculation. Missing serum lipid results for the rest of the 47 patients were found, and the data from those patients were not included from the final analysis. Table I shows baseline characteristics of the study population; the characteristics of the patients in the two groups were similar. The treatment group comprised 324 patients (188 men and 136 women, aged 56.0±0.50 years) and the positive control group comprised 122 patients (73 men and 49 women, aged 56.4±0.83 years). Patients in the two groups had hyperlipidemia for a similar number of years.
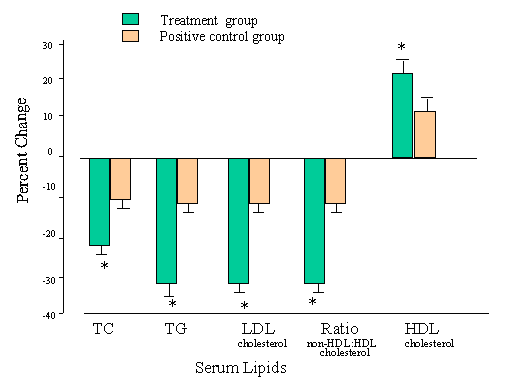
Serum Total Cholesterol
Serum
Triglycerides Serum
High-Density Lipoprotein Cholesterol Ratio
of Non-High-Density Lipoprotein: High-Density Lipoprotein Cholesterol Integrated
Analysis of Effects
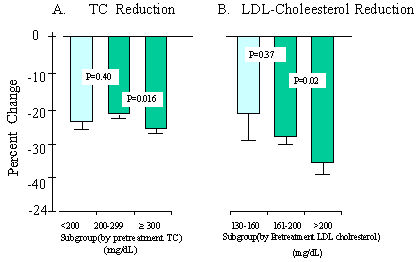
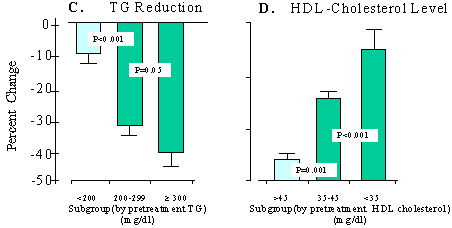
Figure 2A displays a 22% to 26% decrease in TC in the patients with different baseline pretreatment TC (P< 0.001, as compared with the pretreatment baseline). The percentage reduction was similar for the two groups of patients whose pretreatment TC was < 240 mg/dL or =300 mg/dL. However, a nearly twofold reduction in TC (-86 mg/dL) was seen in patients with pretreatment TC=300 mg/dL, as compared with an average reduction of 47.1 mg/dL in patients with pretreatment TC< 240 mg/dL (P< 0.001, comparing the two subgroups). The actual decrease in LDL cholesterol also appeared to be dependent on the level of the pretreatment LDL cholesterol (Figure 2B). However, the percentage reduction of LDL cholesterol did not differ significantly (P=0.11) between the patients with pretreatment LDL cholesterol of 130 to 160 mg/dL (-22.4±7.2%) and pretreatment LDL cholesterol > 200 mg/dL (-34.6±2.1%). The reduction in TG, both actual (mg/dL) and percentage reduction, was more marked when pretreatment TG were highly elevated (Figure 2C). For example, patients with pretreatment TG=300 mg/dL had the largest percentage drop (-40.1%, -152 mg/dL, P< 0.001) from pretreatment baselines compared with the decrease (-8.8%, -17 mg/dL) in those with pretreatment TG< 200 mg/dL (P< 0.001, comparing the subgroups). Pretreatment HDL cholesterol also determined the percentage increase in this lipid (Figure 2D). In patients with HDL cholesterol > 45 mg/dL, M purpureus rice preparation therapy resulted in a minor 4.0% increase in HDL cholesterol (+1.81 mg/dL, P=0.041, compared with pretreatment baseline). In contrast, the percent increase in patients with pretreatment HDL cholesterol of 35 to 45 mg/dL was +16% (+6.3 mg/dL, P< 0.001, as compared with pertreatment baseline); in patients with pretreatment HDL cholesterol< 35 mg/dL, the increase was 25.1% (+7.9 mg/dL, P< 0.001, as compared with pretreatment baseline).
*Clinically
Control = posttreatment serum lipid levels within normal ranges
(total cholesterol [ TC ] <200 mg/dL, low-density lipoprotein
cholesterol [ LDL cholesterol] <130 mg/dL, triglycerides [TG]
<160 mg/dL, and high-density lipoprotein cholesterol [HDL cholesterol]
>40 mg/dL for males and >45 mg/dL for females; Highly Effective
=at least one of the following posttreatment changes in serum lipids:
TC reduced =20%, TG reduced =40%, HDL cholesterol increased=10 mg/dL,
or ratio of non-HDL: HDL cholesterol reduced =20%; Effective = at
least one of the following posttreatment changes in serum lipids:
TC reduced=10% to <20%, TG reduced =20% to <40%, HDL cholesterol
increased =4 mg/dL to <10 mg/dL, or non-HDL cholesterol: HDL
cholesterol reduced =10% to <20%; and Not Effective = posttreatment
serum lipids unchanged or below the criteria for a rating of Effective. Total
Effective rating obtained by adding the numbers of patients whose
outcomes were rated Clinically Controlled, Highly Effective, or
Effective.
Values are mean ± SE of mean. The P values were determined by using Student's t test. Body
Weight Reduction Safety
Assessment DISCUSSION AND CONCLUSIONS This single-masked clinical study involving patients from four clinical sites demonstrated that M purpureus rice preparation administered daily for 8 weeks was very effective in reducing serum cholesterol, with an average 22.7% reduction in TC and 30.9% in LDL cholesterol. The percentage reduction in TC and LDL cholesterol obtained with M purpureus rice preparation was independent of pertreatment levels, but the absolute reduction was greatest in those patients with baseline TC=300 mg/dL or LDL cholesterol > 200 mg/dL. Based on guidelines developed by the Chinese Ministry of Public Health, 16M purpureus rice preparation was effective in 93.2% of the patients and highly effective in 79.6% of the patients treated. In addition to lowering TC and LDL cholesterol, this study showed that M purpureus rice preparation was effective in reducing serum TG in patients with hyperlipidemia. This TG-lowering effect was more evident when pretreatment TG were > 300 mg/dL. There was also a 19.9% increase in HDL cholesterol in patients who received M purpureus rice preparation. This effect was also dependent on the pretreatment baseline, with an inverse relationship between the two variables. Lower pretreatment HDL cholesterol resulted in a greater increase in HDL cholesterol. Notably, the 34.5% decrease in the ratio of non-HDL cholesterol to HDL cholesterol after 8 weeks of treatment with M purpureus rice preparation suggests that there may be a reduced risk of atherosclerosis in patients receiving this therapy. Animal studies have demonstrated that atherosclerosis was significantly reduced in hyperlipidemic rabbits and quail after M purpureus rice treatment.6 The mechanism by which M purpureus rice preparation reduces serum TG and raises HDL cholesterol is not completely understood. This natural food product (original form available in the United States as cholestin3™ [Pharmanex, Inc., Simi Valley, California ]) does, however, contain many nutritional components, such as unsaturated fatty acids, sterols (betasitosterol, campesterol, stigmasterol), proteins, saccharides, isoflavone and its glycosides, saponin and sapogenin, and trace elements such as selenium and zinc, in addition to compounds that inhibit HMG-CoA reductase. 12It should be emphasized that the small quantity (< 14 mg/dL) of total structure-related HMG-CoA reductase inhibitors found in this natural dietary product cannot account for all the lipid-lowering effects of M purpureus rice. Decreased absorption of ingested lipids, reduced very-low-density lipoprotein cholesterol, and/or facilitated removal of very-low-density lipoprotein cholesterol are all events that may also contribute to a reduction in cholesterol and TG and an increase in HDL cholesterol. Interestingly, our data indicate that there was no complete correlation between any of the changes in patients' serum lipids and decreased body weight in either group. Severe side effects with M purpureus rice treatment were rare, and the treatment was well tolerated in this study. Although mild side effects (ie, heartburn, flatulence, and dizziness) were found in a few patients, these symptoms resolved quickly. No changes in alanine aminotransferase were found after the 8-week treatment; mild increases in creatine kinase were found in both groups. Because of regulatory requirements mandated by the Chinese government,15,16 it was not possible to include a placebo control group to establish whether the rise in creatine kinase was related to treatment. However, it is worth noting that in the EXCEL study,17 the placebo control group had an increase in creatine kinase similar to that seen in the group treated with mevinolin. In
summary, we suggest that M purpureus rice preparation is
a highly effective and well-tolerated dietary supplement that can
be used to regulate elevated serum cholesterol and TG. The reductions
in serum TC, LDL cholesterol, and TG, and the increase in HDL cholesterol
are achieved quickly and are clinically meaningful. M purpureus
rice preparation may, therefore, enable people whose serum lipids
are significantly elevated to maintain serum cholesterol and TG
within a normal range.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
©
2013 Asiapharm Biotech Pte Ltd. All rights reserved.
Notices and Legal Disclaimer Information on this web site is provided for informational purposes only and is not a substitute for professional medical advice. You should not use the information on this web site for diagnosing or treating a medical or health condition. You should carefully read all product packaging. If you have or suspect you have a medical problem, promptly contact your professional healthcare provider. Statements and information regarding dietary supplements have not been evaluated or approved by the U.S. Food and Drug Administration. Please consult your healthcare provider before beginning any course of supplementation or treatment. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||